
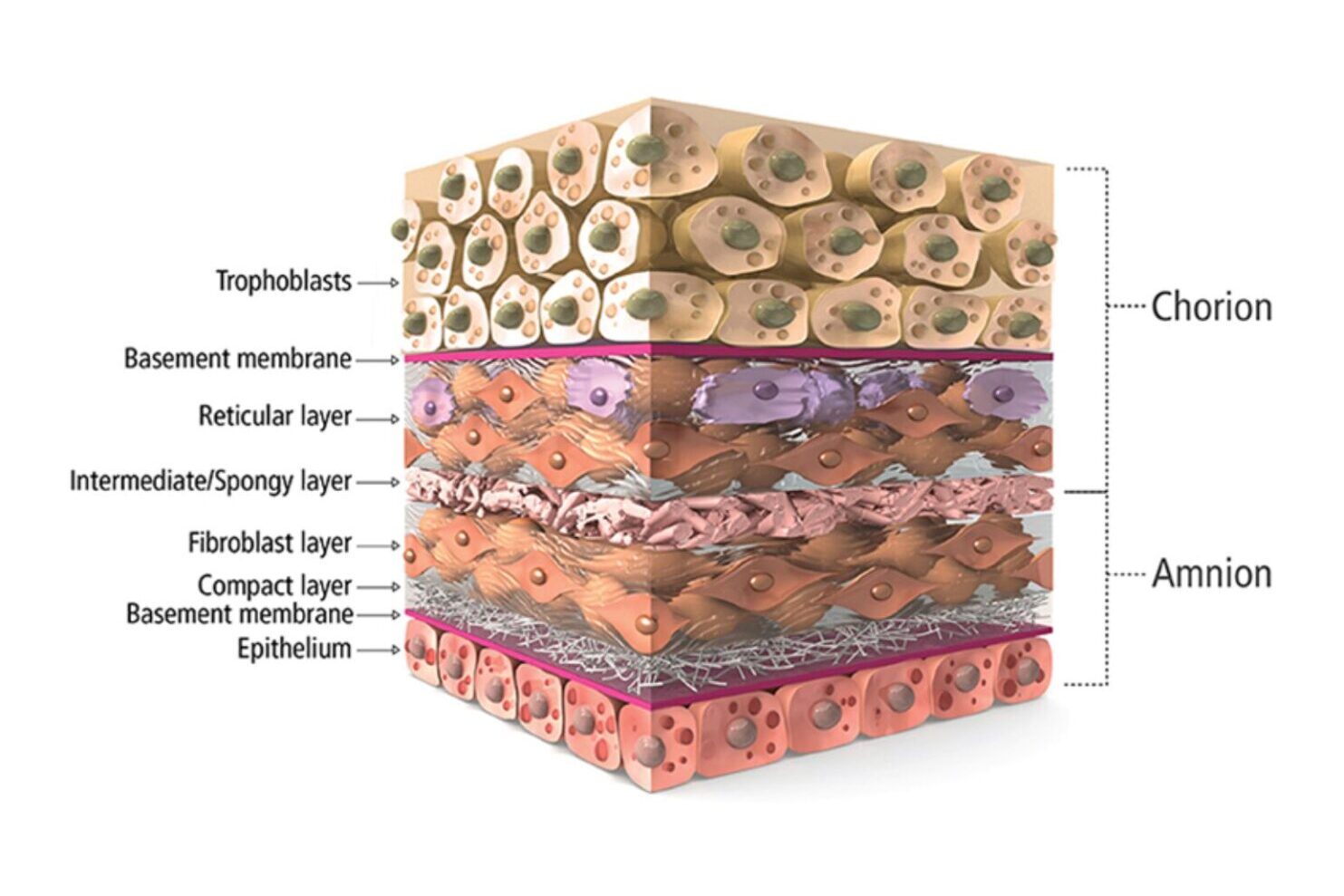
The AmnioPly™ portfolio of grafts are intended to be used as a wound covering to provide a protective barrier from the surrounding environment. These advanced offerings are processed and preserved to retain the biological properties for wound applications.
- Effortless Storage & Usage
- Immune-Privileged
- Various Sizes & Configurations
- Flexible
- Intact Extracellular Matrix
- Aseptically processed in accordance with FDA 21 CFR Part 1271
Available in multiple sizes for application flexibility
AmnioPly™ Dehydrated
| Sizes |
|---|
| 4 x 4 cm | Single, Double, Triple Layer |
| 4 x 6 cm | Single, Double, Triple Layer |
| 4 x 8 cm | Single, Double, Triple Layer |
Amnion-Chorion-Amnion (ACA)
| Sizes |
|---|
| 4 x 4 cm |
| 4 x 6 cm |
| 4 x 8 cm |
AmnioPly™ Cryopreserved
| Sizes |
|---|
| 4 x 6 cm | Single Layer Amnion |
| 7 x 7 cm | Single Layer Amnion |
| 10 x 10 cm | Single Layer Amnion |
| 4 x 6 cm | Single Layer Chorion |
Need to Get in Touch?

